
Education is the cornerstone of biobanking
We promote the quality, the management and the biobanking networks through the a new recognized and up-to-date CAS in biobanking.

Read our related newsletters
Certificate of Advanced Studies
in Biobanking
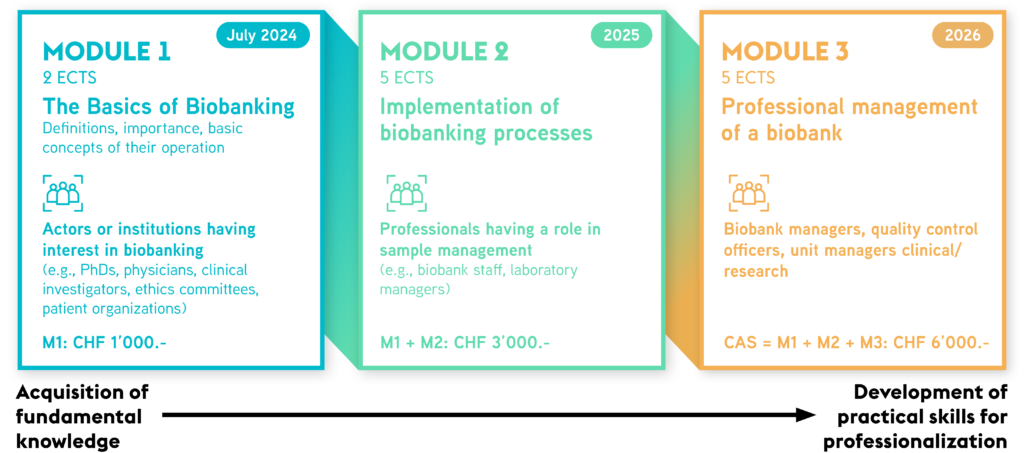
To meet the needs of the different audiences interested in biobanking, we develop in collaboration with different partners (Geneva University, HSeT Foundation, Pasteur Institute), a training course divided into 3 modules. These modules can be followed independently or in succession, in which case the training would lead to a CAS type certification awarded by the University of Geneva.
Introduction
The Certificate of Advanced Studies (CAS) in Biobanking of the Faculty of Medicine at the University of Geneva is an innovative and unique training course in Switzerland and around the world. The aim of the CAS is to train individuals from around the world with an interest in biobanking and research (e.g., PhDs, physicians, clinical investigators, ethics committees, patient organizations). This state‑of‑the‑art advanced training programme is meticulously designed to give participants the indispensable knowledge and proficient skills critical for the successful establishment and management of a biobank. The central purpose is to elevate the standards of quality, management protocols, and interconnections within the domains of clinical laboratory medicine and pioneering research endeavors.




Objectives
Module 1
- Recognize the significance of biobanking in scientific research, healthcare, and advancing medical knowledge.
- Define biobanks based on their unique characteristics, including types (e.g., research, clinical), functions, and the type of samples they handle.
- Understand the ethical and legal regulations governing biobanks, ensuring compliance and responsible sample management.
Module 2
- Prepare required documentation, implement coding processes and consent procedures, and maintain quality standards for proper sample management.
- Recognize the value of local and global biobanking networks, utilizing their resources and collaborating with various stakeholders.
- Establish and oversee biobanks by considering resource allocation, governance structures, operational processes, and quality control systems.
Module 3
- Identify training needs in order to ensure the proper development and management of a biobank.
- Keep informed of local and international ethical and legal requirements related to biobanks, and monitor their future developments.
Audience
Beginners to specialized professionals in Switzerland and abroad, working with biological samples and wishing to acquire harmonized and proper knowledge and competencies in biobanking: medical doctors, biologists, biochemists, pharmacists, researchers, biobank managers, data managers, clinical investigators, ethics committees, patient organisations, etc.
Module 1 is designed for actors (e.g. PhDs, physicians, clinical researchers, ethics committees, patient organisations) having an interest in learning about the basics of biobanking (Beginner)
Module 2 is oriented to participants having followed Module 1 or professionals dealing with biological sample management (Intermediate).
Module 3 targets professionals having followed the previous modules,
such as biobank managers, quality control officers, clinical unit managers (Advanced).
CAS in Biobanking, General Presentation
By Prof. Jean Villard
Head of the Immunology and Transplant Unit, HUG
The Basics of Biobanking (Module 1)
By Dr. Christine Joye
SBP Executive Director
Easy-GCS
The Easy-GCS is an online tool that provides researchers with easy accessible and relevant information – needed for study planning and conduct.
SBP has worked in collaboration with SCTO to develop the biobanking module of the Easy Guide for Clinical studies. The Easy-GCS is a comprehensive tool that provides all professionals involved in clinical studies answers and guidance on how to proceed with the set up and implementation of their study. In practice, the information related to the proper conduct of a clinical trial is accessible through a GRID divided into six study phases (from concept to completion) and eleven study subjects including the one focusing on Biobanking.
The new educational tool including the biobanking module should be available in 2023. This work is a first education program on biobanking within clinical studies and a fruitful collaboration with SCTO.


