Want to get an SBP Label?
Quality strategy
The collected, processed and stored biological samples are important research tools used to perform efficient and high-quality investigations or research. Sample quality is a wide concept, which should be understood as a whole to establish standard control criteria and foster good biobanking practices.
What means Quality Management System for a biobank?
The control and monitoring of resources, assuring the ethical grounds and regulatory requirements, increasing policy improvement and stakeholder confidence. It allows strategic orientation towards performance, to meet the needs of researchers and the expectations of society.
SBP has developed tools and support documents to help biobanks in the establishment and implementation of their quality strategy. This documentation is at the cornerstone of quality and should be part of the Quality Control strategy developed by the biobank to satisfy the ISO 20387 standard requirement (“Biotechnology – Biobanking – General requirements for biobanking”).
« The Quality Management System is the Swiss Army Knife for the development of the biological resources and the know-how of a biobank. »
Jeanne-Hélène di Donato — Biobanks Consultant

Reach a higher level of quality
With this web-based solution, we evaluate the practices of your biobank and bring you to the next level.




The evaluation process
- Biobank registration on Biobank SQAN
- Completion of an online questionnaire with about 250 questions covering
multiple fields of biobanking - Score calculation based on the biobank answers
- Review of answers and key documents (e.g. governance, SOPs)
- Summary of findings into a report and proposal of an action plan and support
documentation for the gaps that were identified
What are the SBP labels?
Proclaim the quality of your biobank at a national level and become compliant with the different minimal requirements.

« I particularly appreciated the first check phase which identified the processes we could improve and therefore facilitated our work in understanding where to focus. »
Dr Irina Banzola – Urology Clinic, University of Zurich
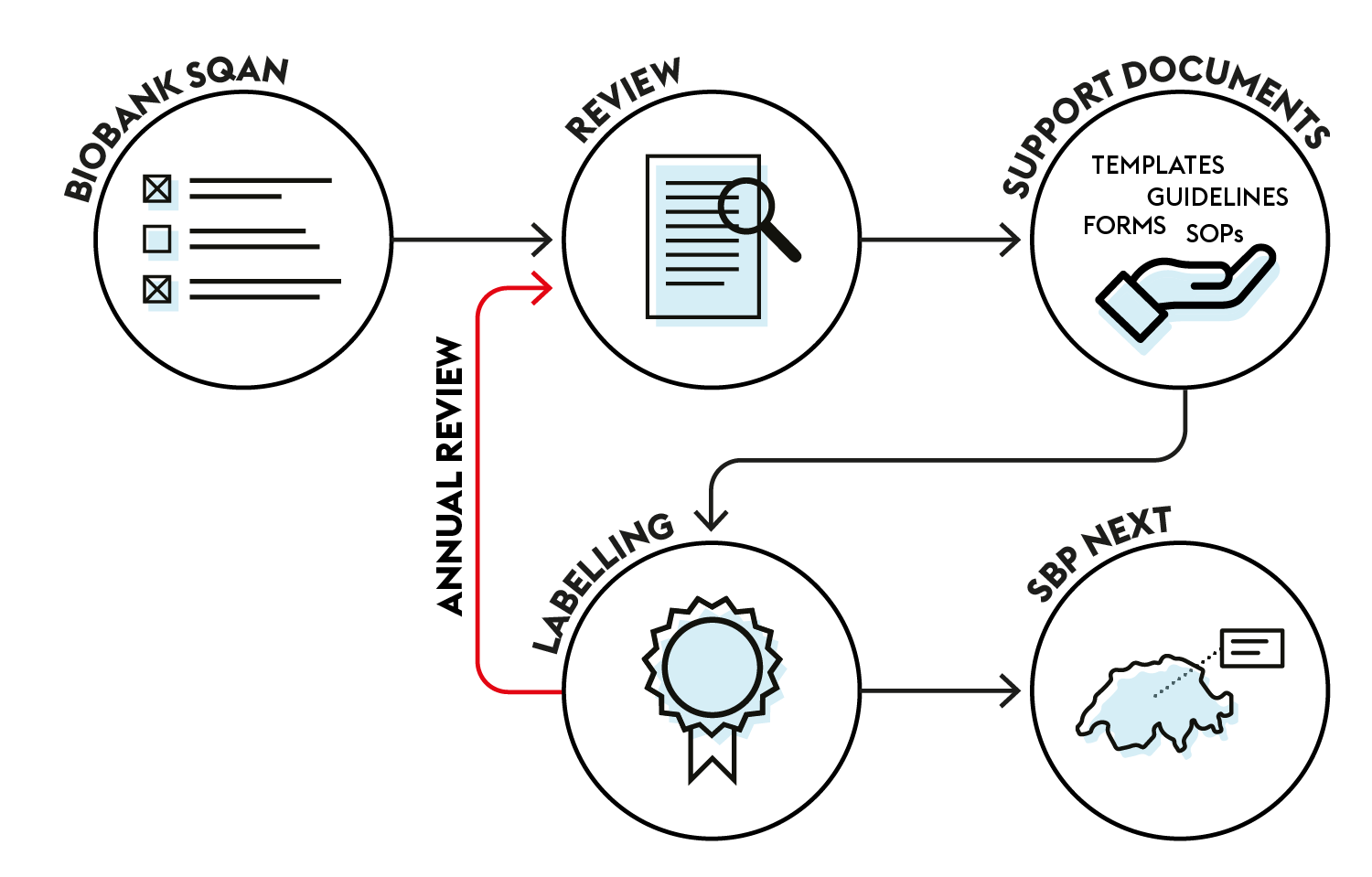
How to obtain a label?
Advance the process toward excellence
When registering on Biobank SQAN, you take the first step on the practice assessment ladder.
Our experts will then support you to progressively obtain compliance with the following minimal requirements.